Semiconductors are materials that have an electrical conductivity somewhere between those of conductors and insulators, i.e., they have a band gap of 4 eV, smaller than that of insulators and larger than that of conductors.
There are two kinds: single-element semiconductors (like silicon or germanium) or compound semiconductors (like gallium-arsenide). Solid-state physics and materials science are interested in the latter. Electrical engineering is most concerned with silicon semiconductors.
Semiconductor physics and silicon wafer fabrication are complicated subjects of their own right. A graduate degree is necessary to make it far in the field. Semiconductors find considerable use in solar photovoltaics, LED lighting, and modern electronics.
Sub-topics
- Doping (extrinsic devices)
- pn junction
Basic types
Semiconductors can be divided into intrinsic and extrinsic semiconductors.
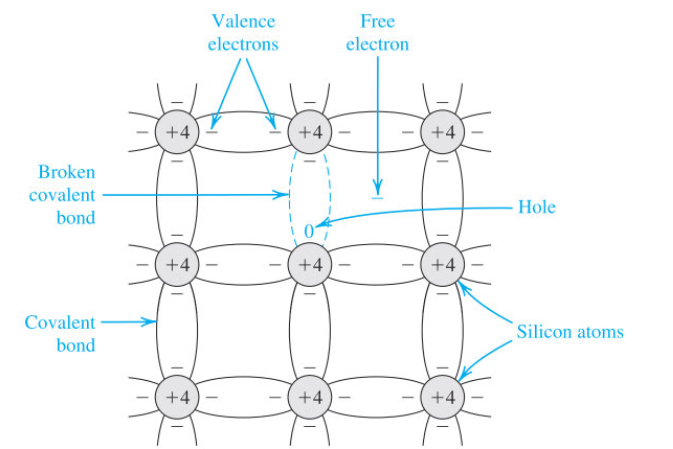
Extrinsic behaviour
Extrinsic semiconductors make use of doping, i.e., we add impurities into the silicon to control its conductivity. The conductivity introduced by the dopant overpowers the intrinsic behaviour.
Two types:
- p-type add holes to the silicon (positive, i.e., remove electrons)
- n-type adds electrons (negative)
Intrinsic behaviour
Intrinsic semiconductors have conductivity controlled by the semiconductor itself, not from what is added to it via doping. In other words, for every electron we promote, we create one hole, i.e., . Its conductivity is given by:
Intrinsic semiconductors aren’t particularly useful, since in practice most semiconductors are doped to better control conductivity. The number of free electrons and holes are given by:1
where is a material-dependent parameter (for silicon, ), is the temperature in Kelvin, is the bandgap energy (also material-dependent, 1.12 eV for silicon), and is the Boltzmann constant.
Characteristics
Semiconductors have several key electrical properties:
Footnotes
-
From Microelectronic Circuits by Sedra/Smith. ↩