First examined in high school physics.
Decay
Most nuclides are unstable, where they randomly and spontaneously emit particles that carry energy away from the nucleus. Random means that we cannot predict which unstable nucleus in a sample will decay or when there will be a decay. Spontaneous means we cannot affect the rate of decay of a given sample in any way. We do know that the number of nuclei that will decay per second is proportional to the number of nuclei that have not yet decayed (the law of radioactive decay). In general, as the atomic number increases, so too do the numbers of neutrons relative to the number of protons in stable nuclei. This is explained by the strong force, since neutrons will contribute to nuclear binding through the strong force but won’t add to repulsive forces. Emission of particles and energy are called radioactivity, where emissions of alpha particles, beta particles, and gamma rays take place.
Note! Charge and mass are conserved, so the left and right sides of the reaction equations should balance out.
In alpha decay, alpha particles are emitted from the nucleus and the decaying nucleus turns into a different nucleus. Take the example below where uranium alpha decays into thorium. Note that alpha particles are identical to helium nuclei.
In beta minus decay, a neutron in the decaying nucleus turns into a proton, emitting an electron and an anti-neutrino. Beta minus particles are electrons. In beta plus decay, the nucleus instead emits the electron’s anti-particle, the positron.
In gamma decay, the nucleus emits a gamma ray, i.e., a photon of high-frequency electromagnetic radiation. The nucleus remains the same.
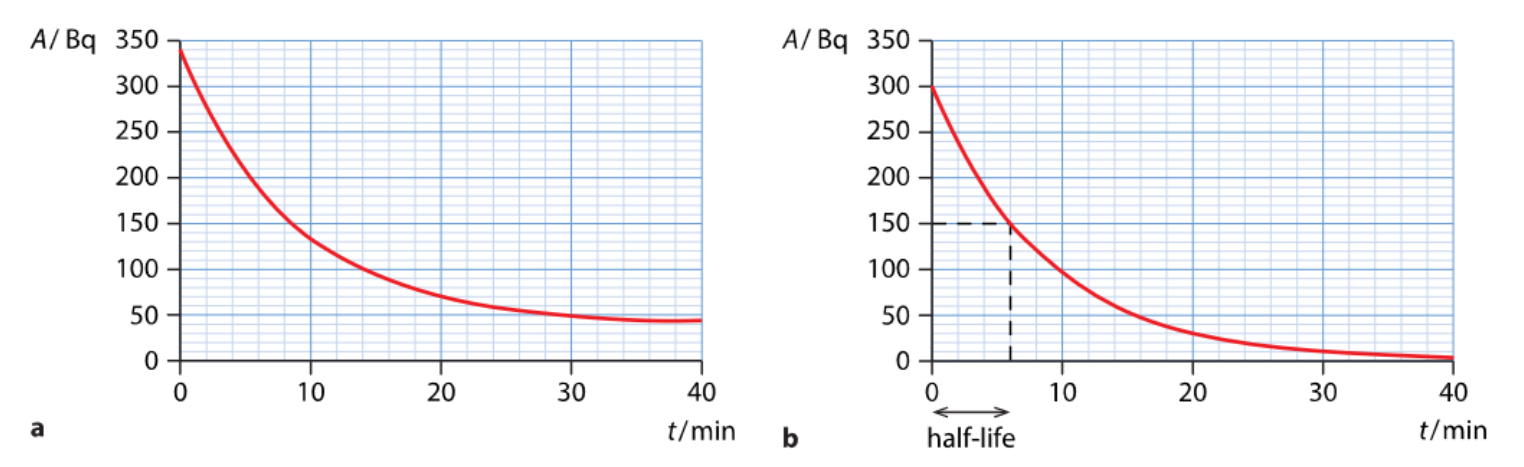
Returning to decay as a whole, we introduce two new terms: half-life and activity. We define half-life as the time after which the number of radioactive nuclei is reduced by a factor of 2. Activity is the number of decays per second, measured in becquerels (Bq).
Binding energy
To separate protons and neutrons, we must supply energy to the nucleus. Conversely to separate nucleons, we must also release energy.